Question
Does insulin resistance specifically increase risk of Alzheimer’s disease and/or neurodegeneration? If so, how?
Takeaway
This study found increased insulin resistance was associated with lower glucose metabolism in the brain, specifically in regions associated with memory function, and this reduced metabolism was associated with memory impairment. These results are consistent with a larger body of evidence indicating insulin resistance (as seen in diabetes and the metabolic syndrome) is at minimum a risk factor for Alzheimer’s disease and may play a direct causal role.
The ADA estimates more than 29 million Americans, and more than half of those over age 64, are diabetic or prediabetic (1). Insulin action, in both the peripheral and central nervous systems, stimulates glucose uptake and utilization and promotes microvascular blood flow. Previous human and animal research has shown impaired insulin signaling in the brain is associated with brain atrophy, mitochondrial dysfunction, inflammation, memory deficits, reduced cerebral blood flow, and cognitive deficits (2).
As previously discussed on CrossFit.com, these deficits may be related to deficient cerebral glucose metabolism. Impairments in brain glucose metabolism, particularly in regions of the brain associated with memory and executive function, are a consistent feature associated with mild cognitive impairment (MCI) and Alzheimer’s disease (AD); the same impairment is also seen in individuals with the APOE e4 allele (a major genetic risk factor for AD) even when healthy (3).
This observational study sought to understand the link between peripheral insulin resistance — the type seen in prediabetic and diabetic individuals — and reduced brain glucose metabolism and memory function. One hundred fifty middle-aged (age 40-65) adults were recruited from the Wisconsin Registry for Alzheimer’s Prevention. All were cognitively normal. Each subject was assessed for insulin resistance, brain glucose metabolism (via FDG-PET), and memory function.
Higher HOMA-IR levels — i.e., higher levels of insulin resistance — were associated with reduced glucose metabolism throughout the brain (Figure 1). The greatest suppression of glucose metabolism was seen in the medial temporal lobe (MTL) and hippocampus, areas linked to memory function. The degree of insulin resistance was proportional to the degree to which glucose metabolism was suppressed. These two factors were additionally associated with lower scores in various tests of memory function.
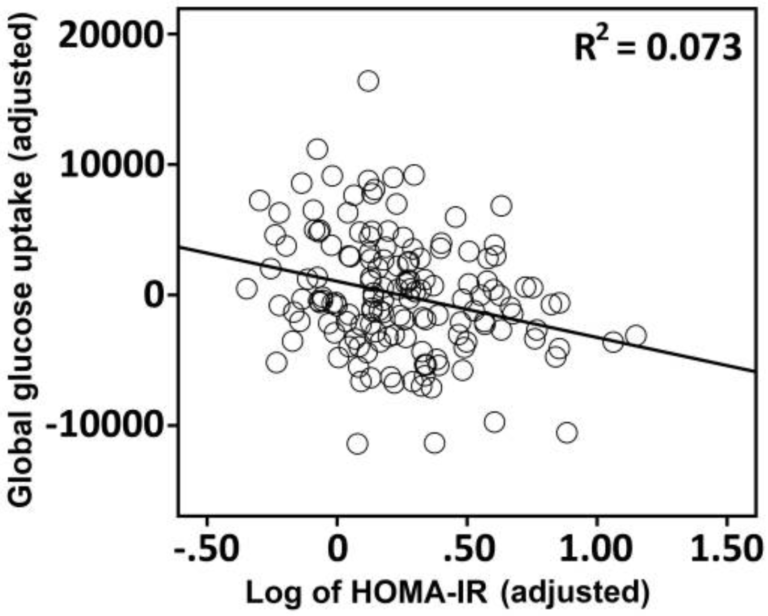
Figure 1: The association between HOMA-IR and global FDG-PET uptake in 150 late middle-aged adults.
The results of the study are consistent with previous work showing peripheral insulin resistance, as well as elevated fasting glucose and insulin levels, are associated with impaired brain glucose metabolism (4). The observed link between insulin resistance, decreased glucose metabolism, and impaired memory provides a potential mechanism for the observed associations between other forms of metabolic disease and Alzheimer’s and supports further efforts to explore this hypothesis (5). As previously reviewed on CrossFit.com, and as noted by the authors of this study, insulin resistance may impair cognitive performance through other mechanisms not directly related to glucose metabolism, including decreased mitochondrial function, oxidative stress, inflammation, and dysregulated lipid metabolism (6).