Dr. Robert Lustig, a pediatric neuroendocrinologist, has long been on the cutting edge of medicine and science. Throughout his forty-plus year career, The New York Times bestselling author of Fat Chance has been dedicated to treating and preventing childhood obesity and diabetes. With his new book, METABOLICAL: The Lure and the Lies of Processed Food, Nutrition, and Modern Medicine (Harper Wave; May 4, 2021; $28.99), he will expose the truth, both scientifically and politically, underlying the current global pandemic of diet-related diseases.
In his previous work, The Hacking of the American Mind, Dr. Lustig elucidated how the processed food industry has hacked our bodies and minds to pursue pleasure over happiness, fueling widespread addiction and depression. In METABOLICAL, he addresses nutrition, food science, and global health, and explains how by focusing on real food we can reverse chronic disease and promote longevity. For the first time, all strands of this pandemic — the medical, the economic, and the environmental — are pulled together into one clear narrative.
Describing the eight pathologies within the cell that belie all chronic disease, Dr. Lustig illustrates how they are not “druggable” but rather “foodable” (i.e. medication can’t cure what nutrition can) by following two basic principles: protect the liver, and feed the gut. He uses this science to chronicle the breakdown in our current healthcare paradigm, which has succumbed to influence from Big Food, Big Pharma, and Big Government. In the special chapter “Food in the Time of Corona,” Dr. Lustig addresses the way “pre-existing conditions” (i.e. diet-induced chronic diseases) make us vulnerable to succumbing to acute infectious diseases like COVID-19. He also argues that the Nutrition Facts label hides information from the consumer by omitting what’s been done to the food, which is more important than what’s in the food.
Weaving together the interconnected strands of nutrition, disease, medicine, environment, and society, METABOLICAL provides the scientific bases for a series of iconoclastic revelations, among them:
- Medicine for chronic disease only treats symptoms, not the disease itself
- You can diagnose your own biochemical profile
- Processed food isn’t just toxic, it’s addictive
- The war between vegan and keto is a false war—the combatants are on the same side
- Big Food, Big Pharma, and Big Government are on the other side
METABOLICAL will be available for purchase on May 4, 2021. Blurb and excerpt courtesy of Harper Wave.
Chapter 8. Checkpoints Alpha, Bravo, Charlie: nutrient-sensing and chronic disease
PART 3 (SEE PART 1 AND PART 2)
Growth versus burning, and everything in between — the 8 permutations
Based on these precepts of cell biology, I offer you a new way to think about the role of diet and nutrition in the development of NCD’s. This is a hypothesis, not proven — but it fits the available scientific data and the effects of nutrients on growth, burning, and disease. These three enzyme checkpoints explain how the cell metabolizes energy: 1) PI3-kinase imports glucose into the cell; 2) AMP-kinase directs the energy to mitochondria for burning; and 3) mTOR determines whether a cell lives or dies. They explain health and longevity when working in harmony, but also explain the subcellular pathologies (see Chapter 7) and NCD’s when they are dyssynchronous and working at crossed purposes. Three checkpoints with two valences (+ or —) each total 2^3 or eight possible permutations, listed in Table 8-1. One constraint is that AMP-kinase trumps mTOR — when AMP-kinase is turned on, mTOR is turned off. Growth is listed in Column 1, Burning in Column 2 – both of which are needed for the cell to survive. Any of the other permutations (Columns 3-8) occur due to an energy “fork-in-the-road”, and can lead to one or more pathologies, which if unchecked could foment different types of NCD’s. We don’t know that the last two actually occur (because AMP-kinase turns off mTOR consistently) but they make sense to include for completeness. Each of these permutations are subject to dietary manipulation, either for good or for bad. Scenarios for each are listed below.
Table 8-1. The activity of three enzymes (PI3-kinase, AMP-kinase, and mTOR), in two different states (high (+) or low (—)), leads to eight separate permutations. In any given cell at any given time, each enzyme can either be (+) or (—), although AMP-kinase (+) activation automatically results in mTOR (—) inactivation; thus permutations 7 and 8 are theoretical.
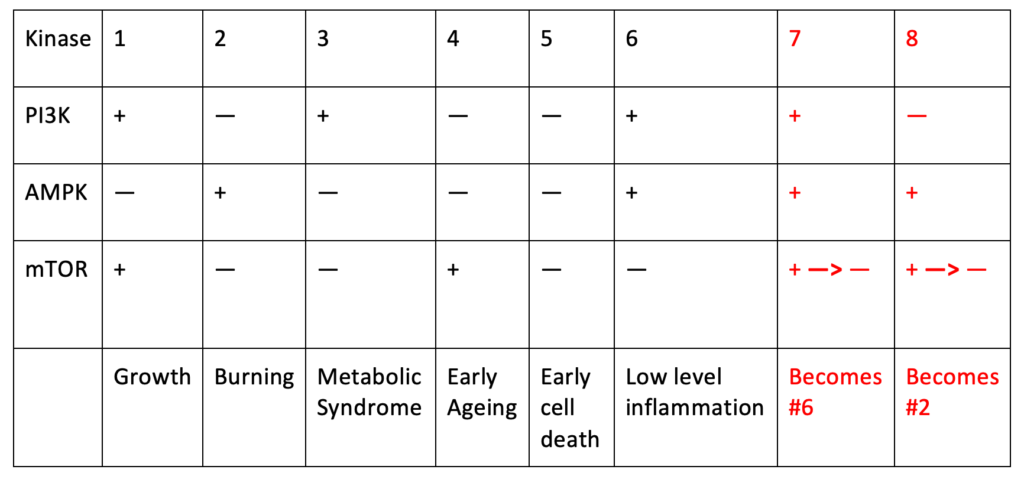
1. PI3K +, AMPK –, mTOR +
This permutation leads to growth, which occurs in the absence of oxygen. When PI3-kinase and mTOR are turned on and AMP-kinase turned off, cells will import lots of glucose, and use it to make lipids for membranes, amino acids for proteins, and ribose for DNA. This increases glycation and oxygen radicals. It can also increase the risk for cancer; every time a cell divides there is a chance that a mistake will be made in the DNA copying, which could lead to mutations that cause cancer.
2. PI3K –, AMPK +, mTOR –
This permutation leads to burning, and occurs in the presence of oxygen. The goal is ATP generation with carbon dioxide as a byproduct. Because PI3K is turned off, glucose will be in low supply, thus glycation and oxygen radicals will be low. Since mTOR is turned off, autophagy is likely. More AMP-kinase means more and healthier mitochondria, and risks for metabolic syndrome and cancer development are low.
3. PI3K +, AMPK –, mTOR –
This permutation leads to classic metabolic syndrome. Glucose enters the cell but because mitochondria are not activated, it has nowhere to go. Glycation and inflammation will increase. Even though mTOR is turned off, the glucose supply will mean that the cell will likely not die of autophagy. Insulin will be high, driving production of lipids/fat. The end result is muscle fat, liver fat, and insulin resistance, obesity due to the high insulin, and eventually type 2 diabetes. And processed food is the obvious driver.
4. PI3K –, AMPK –, mTOR +
This permutation is likely to lead to early aging. Without glucose flooding the cell, glycation and oxidative stress is low and cell damage will be slow. Without AMP-kinase, mitochondria will not be generating oxidative stress. But the cell is prone to accumulate damage over time because mTOR is turned on, as there is no autophagy. There would be slow development of liver and muscle fat, and possibly cancer.
5. PI3K –, AMPK –, mTOR –
This permutation is likely to lead to early cell death. Less glucose is imported, but it’s not being burned; and there is also increased autophagy. The cell is likely to die easily for quicker turnover, which isn’t a bad thing metabolically, and very little risk for cancer.
6. PI3K +, AMPK +, mTOR –
This permutation is likely to lead to low-level inflammation. It is similar to (5), but with more autophagy, so less long-term damage.
7. PI3K +, AMPK +, mTOR + becomes –
This is similar to (6). This permutation is likely to lead to high-level vascular injury and heart disease. Increased glucose entering the cell means glycation and oxidative stress. The glucose will be burned by mitochondria; as AMP-kinase partially inhibits mTOR, there will be some but not complete autophagy, and some clearing of dead cells. This could result in heart disease, but lower risk for cancer.
8. PI3K –, AMPK +, mTOR + becomes –
This is similar to (2), and should lead to burning, and only in the presence of oxygen. Not much glucose, and the burning is aerobic, so not much oxidative stress, and mTOR reduced.
As you can see, these various permutations of the three enzymes impact on the eight subcellular pathologies in Chapter 7, to either cause the cell to grow, burn, or create disease. So how does this model stack up with the data in the literature? One way to assess the veracity of the model is to look at the effects of specific drugs of these enzymes on the cell and the organism. We have data on PI3-kinase inhibitors, AMP-kinase stimulators, and mTOR inhibitors at our disposal, which demonstrate reduced cancer growth (20, 21-23) and increased longevity (24-26), supporting this model.
The scourges of chronic disease — metabolic syndrome, aging, and cancer — are all about how energy is handled at these three checkpoints. And each checkpoint, which drives glycolysis and the Krebs Cycle, is modulated by your diet. There is no current blood test to measure these checkpoints, glycolysis or the Krebs cycle. So what can your doctor’s tests tell you about your health? Chapter 9 will show you how you can use the information your doctor gleans from standard test in order to diagnose yourself, and take charge of your own health.
- D.C. Wallace. “Mitochondria and Cancer: Warburg Addressed,” Cold Spring Harb. Symp. Quant. Biol. 70 (2005): 363.
- J. Lloyd-Price et al. ” The Healthy Human Microbiome,” Genome Med. 8 (2016): 51.
- R.H. Lustig et al. “Isocaloric Fructose Restriction and Metabolic Improvement in Children with Obesity and Metabolic Syndrome,” Obesity 24 (2016): 453.
- M.S. Conceição and C. Ugrinowitsch. “Exercise with Blood Flow Restriction: An Effective Alternative for the Non-Pharmaceutical Treatment for Muscle Wasting,” J. Cachexia Sarcopenia Muscle 10 (2) (2019): 257.
- R.J. Shaw and L.C. Cantley. “Decoding Key Nodes in the Metabolism of Cancer Cells: Sugar & Spice and All Things Nice,” F1000 Biol Rep 4 (2012): 2.
- B.D. Hopkins et al. “Suppression of Insulin Feedback Enhances the Efficacy of Pi3k Inhibitors,” Nature 560 (2018): 499.
- C. Greenhill. “Suppressing Insulin Feedback to Improve Efficacy of Cancer Therapeutics,” Nat. Rev. Endocrinol. 14 (9) (2018): 501.
- A. Woods et al. “Liver-Specific Activation of Ampk Prevents Steatosis on a High-Fructose Diet,” Cell Rep. 18 (13) (2017): 3043.
- B. Viollet et al. “Cellular and Molecular Mechanisms of Metformin: An Overview,” Clin. Sci. 122 (2012): 253.
- A. Gugliucci. “Fructose Surges Damage Hepatic Adenosyl-Monophosphate-Dependent Kinase and Lead to Increased Lipogenesis and Hepatic Insulin Resistance,” Med. Hypotheses 93: 87.
- A. Erkin-Cakmak et al. “Isocaloric Fructose Restriction Reduces Serum D-Lactate Concentration in Children with Obesity and Metabolic Syndrome,” J Clin Endocrinol Metab. 2019; 104 (7) (2019): 3003.
- R.A. Saxton and D.M. Sabatini. “Mtor Signaling in Growth, Metabolism, and Disease,” Cell 168 (2017): 960.
- J.B. Mannick and e. al. “Mtor Inhibition Improves Immune Function in the Elderly,” Science Transl. Med. 6 (2014): 268ra179.
- M. Perluigi et al. “Mtor Signaling in Aging and Neurodegeneration: At the Crossroad between Metabolism Dysfunction and Impairment of Autophagy,” Neurobiol. Dis. 84 (2015): 39.
- D.M. Sabatini. “Twenty-Five Years Obsessing over Mtor,” Proc Natl Acad Sci U S A. 114 (45) (2017): 11818.
- T. Weichhart. “Mtor as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review,” Gerontology 64 (2) (2018): 127.
- S. Sengupta et al. “Regulation of the Mtor Complex 1 Pathway by Nutrients, Growth Factors, and Stress,” Mol. Cell 40 (2010): 310.
- P. Nicklin et al. “Bidirectional Transport of Amino Acids Regulates Mtor and Autophagy,” Cell. 136 (2009): 521.
- Y. Li et al. “Tsc2: Filling the Gap in the Mtor Signaling Pathway,” Trends Biochem. Sci. 29 (2004): 32.
- J. Yang et al. “Targeting Pi3k in Cancer: Mechanisms and Advances in Clinical Trials ” Mol. Cancer 18 (1) (2019): 26.
- G. Muscogiuri et al. “The Crosstalk between Insulin and Renin-Angiotensin-Aldosterone Signaling Systems and Its Effect on Glucose Metabolism and Diabetes Prevention,” Curr. Vasc. Pharmacol. 6 (2008): 301.
- K.N. Shah and S.S. Patel. “Phosphatidylinositide 3-Kinase Inhibition: A New Potential Target for the Treatment of Polycystic Ovarian Syndrome,” Pharm. Biol. 54 (6) (2016): 975.
- D.R. Morales and A.D. Morris. “Metformin in Cancer Treatment and Prevention,” Ann.Rev. Med. 66 (2015): 17.
- N. Barzilai et al. “Metformin as a Tool to Target Aging,” Cell Metab. 23 (2016): 1060.
- Z. Zou et al. “Targeted Inhibition of Rictor/Mtorc2 in Cancer Treatment: A New Era after Rapamycin,” Curr. Cancer Drug Targets 16 (2016): 288.
- N. Duval et al. “Rapamycin Treatment Ameliorates Age-Related Accumulation of Toxic Metabolic Intermediates in Brains of the Ts65dn Mouse Model of Down Syndrome and Aging,” Front. Aging Neurosci. 10 (2018): 263.

Metabolical: The Lure and the Lies of Processed Food, Nutrition, and Modern Medicine — Excerpt 3