Long gone are the days of finishing dinner at 6:00 p.m. and waiting to eat until breakfast the next day at 8:00 a.m. In 2016, Shubhroz Gill and Satchidananda Panda of the Salk Institute, conducted a study in which participants logged their food intake using a smartphone app. Everything they ate was photographed, time stamped, and sent to the researchers. More than half the people studied had an eating window greater than 14 hours and 45 minutes. The average person in the study had their first meal of the day within an hour of waking and their last meal within two hours of bedtime. It’s an almost perfect reversal of the eating patterns of yore.
Metabolic States
In the previous installment, we looked at meal frequency. This was an area of great research interest after epidemiological studies showed clear trends between higher meal frequency and improved health outcomes. Controlled studies showed meal frequency to be far less important than expected. What is now becoming clear is the importance of the time spent not eating. The time from your first meal of the day to your last is called the eating window, and the rest of the time (including while you are asleep) is called the fasting window.
After eating a meal, we are in what is known as the fed state. For the next several hours, our energy needs are met by the food we are digesting. When we are not eating, we are in the fasted state. In this state, energy must be drawn from our glycogen and fat reserves. Insulin levels drop, allowing access to this stored energy, sustaining us until our next meal. Generally, we first draw upon our glycogen stores and then fat stores. It was once feared that skipping a meal would initiate “starvation mode,” and protein from our muscles would be catabolized for energy. We’ll explore recent research that shows this fear to be misguided.
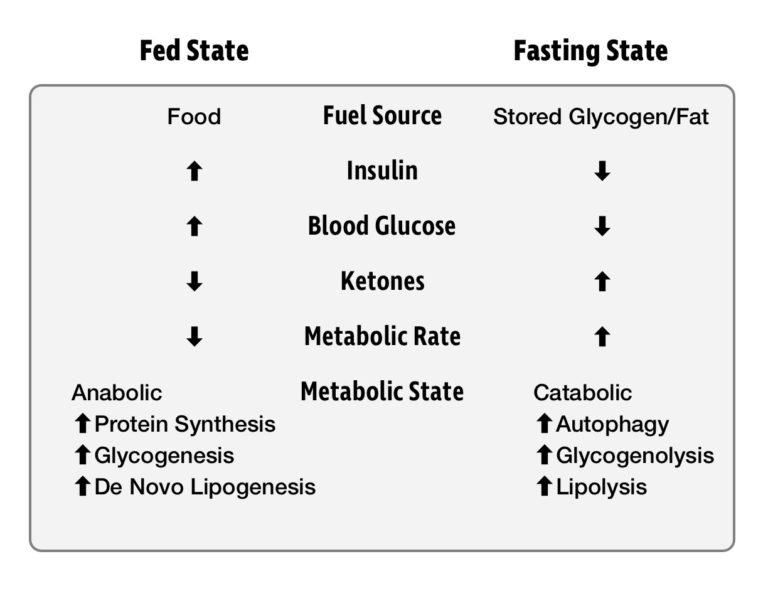
An important thing to know about energy metabolism is that it never stops. Our body, and especially brain, needs energy 24 hours a day, seven days a week. Death can occur within four minutes if blood glucose drops too low, but our metabolism is incredibly effective at preventing this from happening. When blood glucose goes too high and becomes toxic, insulin drives the excess glucose into storage. When we are deprived of food and blood glucose drops too low, the hormone glucagon raises it. Our metabolic machinery maintains blood glucose within a tightly regulated window in which glucose remains not too low to kill us and not so high as to poison us.
The fact that food intake is not continuous poses a major challenge for our metabolism. There is no way for us to stay within a tight blood glucose range by grazing all day. In nature, there’s no guarantee that your next meal will be available the moment you want it. To allow us to survive through the fasting window, our body has several sources of stored energy. Glycogen stored in the liver and muscles is sufficient to last a day, and fat in our adipose tissue can last for several weeks. A metabolically healthy person has no problem tapping into both stores of energy when needed.
Unfortunately, the abundant fat stores of an obese person are trapped behind an insulin barrier. There’s plenty of fat available, but chronically elevated insulin (hyperinsulinemia) keeps it in storage. Normally, fat released from storage keeps hunger at bay in the fasted state. Obese individuals struggle to switch their metabolism to fat burning even while fasting. Frequent eating driven by constant hunger keeps such a person trapped in the fed state. This might require a more powerful intervention than short-duration, intermittent fasts.
Entering the Fasted State
A prolonged absence of food gives our digestive system a chance to rest and do cleanup. It can take many hours after your last meal to complete digestion and enter the fasted state. The time varies between individuals, but 12 hours is often suggested as an estimate.
In the fasted state, a cellular energy sensor called AMPK is activated. This enzyme regulates energy homeostasis by increasing fuel availability through lipolysis and glycogenolysis. At the same time, protein synthesis and other energetically costly anabolic processes are shut down. The downstream effects of AMPK increase energy supply and reduce energy demand. Not only is AMPK a master metabolic switch, but it also induces autophagy, or “self-eating” — i.e., the body’s method for clearing out damaged cells and regenerating healthy ones.
Bits of cellular debris, like tangled proteins and damaged organelles, accumulate in your body. Autophagy recycles this garbage into building blocks and energy substrates. Dysfunctional mitochondria are recycled in a process called mitophagy. Healthy new mitochondria are then created, which improves metabolic efficiency. In conditions of food scarcity, this is a very useful adaptation.
Without autophagy, this junk would accumulate in our bodies. In fact, a chronic lack of autophagy has been associated with several health issues. This is evident in the buildup of amyloid-β peptide in Alzheimer’s and α-synuclein in Parkinson’s disease. Authophagy also plays a critical role in the innate immune system. Autophagic degradation of intracellular pathogens — such as viruses, bacteria, and parasites — is called xenophagy.
Autophagy also plays a key role in regulating inflammation. This is described in a paper by Mark Mattson et al. (2016):
All major diseases, including cardiovascular disease, diabetes, neurodegenerative disorders, arthritis, and cancers involve chronic inflammation in the affected tissues and, in many cases, systemically (59). Local tissue inflammation involves hyperactivation of macrophages (microglia in the brain) which produce proinflammatory cytokines (TNF, IL-1β, IL-6) and reactive oxygen species. Overweight and obesity promote inflammation, and IER suppresses inflammation in human subjects and animal models of diseases. Obese women who changed their diet from multiple daily meals to alternate-day energy restriction exhibited significant reductions in levels of circulating TNF and IL-6 (60).
Frequent eating and high caloric intake prevent the body from entering a fasted state where autophagy can do its work, removing garbage and maintaining cellular quality control. Studies in mice have shown dysfunctional autophagy reduces the quality of muscle and impairs function over time. Spending some time in a catabolic state is not the bogeyman bodybuilders once thought.
Time-Restricted Eating
In our grandparents’ generation, a fasting window of 12-14 hours was so normal there wasn’t even a word for it. Today, this way of eating is so uncommon it had to be given a name: “time-restricted eating” (TRE), also commonly known as “time-restricted feeding.”
Many time-restricted eating protocols exist. The most popular is 16:8, meaning you fast for 16 hours a day and eat within an eight-hour window. This method was popularized by bodybuilder Martin Berkhan, inventor of the LeanGains protocol. More extreme variations are 20:4 (a 20-hour fasting window) and the one-meal-a-day (OMAD) plan.
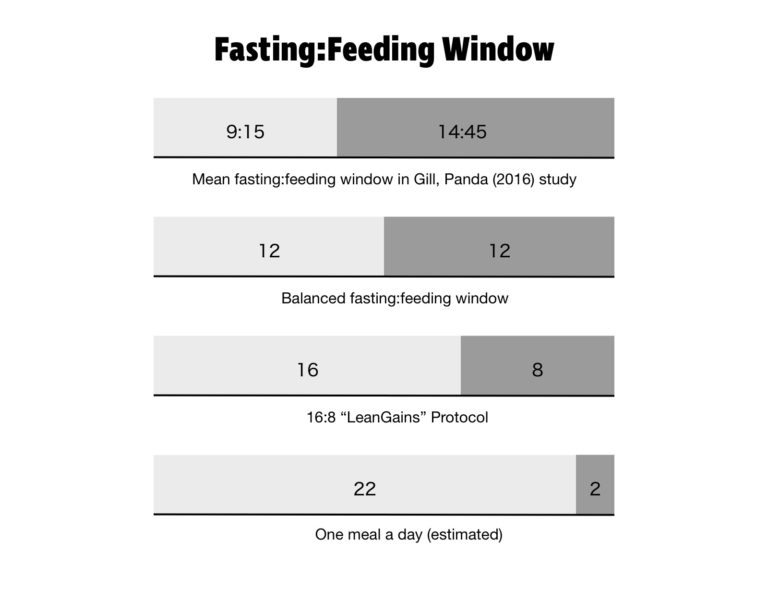
There are a variety of other eating schedules to choose from that increase time spent in the fasted state. Alternate-day fasting involves eating normally one day and completely fasting the next day. Fasts lasting 24 or 36 hours are still within the realm of intermittent fasting, but even longer fasts of three to seven days are being utilized in therapeutic protocols for obesity and diabetes.
Many people also find intermittent fasting to be very convenient. The less time a day you are eating, the less you have to worry about food. By skipping breakfast, it’s possible to start your day quickly and not worry about food. Most people do not find hunger to be an issue after an adaptation period. Interestingly, our appetite is tuned to our eating habits by ghrelin entrainment. Prior to meal time, this hunger hormone increases in anticipation of a meal. It can be retrained to a new schedule. To decrease discomfort, it can help to expand your fasting window gradually.
Eating a low-carb diet helps curb hunger when paired with any type of intermittent fasting. Protein and fat stimulate satiety hormones, so we feel more full after a meal and stay satiated longer. Intermittent fasting also requires a switch to fat burning once glucose runs low. If you are already fat-adapted from eating a low-carb diet, this switch is already flipped. Some low-carb eaters report so little hunger while intermittent fasting that they forget to break fast and eat several hours later than planned.
Training and the Fasting Window
Let’s face it, America is not suffering from an abundance of bodybuilders. Our overweight population is almost entirely overfat, not over-muscled. As such, most studies on intermittent fasting are concerned with weight loss. However, a few studies focusing on trained lifters provide evidence that intermittent fasting is a potent method to lose fat while maintaining muscle mass.
You might be familiar with the bulking and cutting method used by bodybuilders. To reach a target body weight, they overshoot the mark in the bulking phase. In the cutting phase, they lose as much fat as possible while maintaining muscle. This cycle of anabolic (bulking) and catabolic (cutting) phases can last for several weeks each. The end goal is to maximize muscle and minimize fat on a specific date.
Intermittent fasting compresses the anabolic/catabolic cycle into a single day. It might not be optimal for the extreme needs of competitive bodybuilders, but evidence is now showing that restricting your eating window can improve your body composition. A major fear of bodybuilders is that fasting will lead to muscle loss, but studies on long-term fasting show that growth hormone is greatly increased, which both spares muscle and further increases fat utilization. Research on time-restricted eating bears this out.
In a 2016 study, 34 resistance-trained men were divided into two groups: the time-restricted feeding (TRF) group fasted 16 hours a day and ate for eight hours, and the normal diet (ND) group fasted and ate for 12 hours each. Both groups were matched for calories and macros (54% carbs, 23% fat, 22% protein) and followed a standardized weight training program three days/week. CrossFit would not approve of either the dietary macros or the training program (a split routine done on machines), but neither group had an advantage over the other.
Both groups maintained their muscle mass over the eight-week study, but the TRF group lost 3.5 lb. of fat, dropping them from 13% to 11% body fat. There was no significant difference in calories consumed between the two groups, so any fat loss was the result of a metabolic advantage. The researchers proposed that one explanation for this is:
… the increase of adiponectin that interacts with adenosine 5′-monophosphate-activated protein kinase (AMPK) and stimulates Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) protein expression and mitochondrial biogenesis. Moreover, adiponectin acts in the brain to increase energy expenditure and cause weight loss [53]. It is notable that in the present study, the differences in adiponectin between groups remained even when normalized relative to body fat mass, whereas the significant decrease of leptin (that might be considered an unfavorable factor for fat loss) was no longer significant when normalized for fat mass.
Translation: Elevation of a hormone called adiponectin increased fat burning via AMPK and its downstream effects on other hormones and gene transcription factors.
Several health biomarkers were improved in the TRF group. Fasting insulin, blood glucose, and several metrics of inflammation (IL-6, TNF-α, IL-1β) were reduced. HDL-C was slightly increased, and triglycerides were slightly reduced, indicating a favorable improvement in metabolic health.
The same research group performed a similar study of time-restricted feeding in 2009 with women. The results were similar to the prior study of men. The women were only advised on when to eat and to maintain sufficient protein intake. Analysis of their diet logs revealed macronutrient ratios of 40% carbs, 27% protein, and 33% fat.
Both the TRF and normal diet groups gained similar amounts of muscle mass (+2-3%), but subjects in the TRF group decreased their body fat by 2-4%, whereas the normal diet group gained 2% body fat. A third group, TRF(HMB), took an exogenous ketone supplement in addition to the time-restricted feeding protocol. No significant differences were found in the TRF(HMB) group.
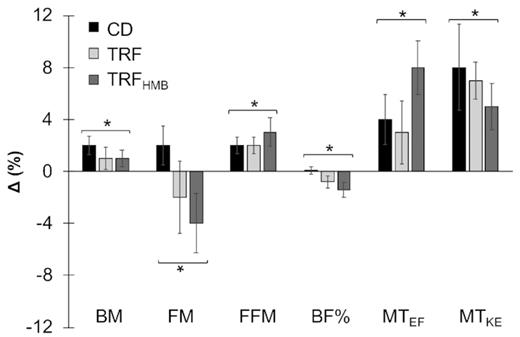
What is of interest in both of these studies is that lean mass was maintained while fat mass was reduced in the intermittent fasting protocol. Importantly, dietary protein intake was controlled in these studies, unlike a previous study in which participants in the control group ate far more protein than the TRF group and gained 2.3 kg of muscle mass. The TRF group maintained muscle mass, but it’s unknown whether they failed to gain muscle mass because of the time-restricted feeding or the insufficient protein intake (1). Furthermore, food intake was reported by questionnaire, which is highly suspect, and outcomes were all over the map.
The previously mentioned studies examined healthy, well-trained subjects engaged in resistance training. One study has tested intermittent fasting on an obese population using an alternate-day fasting (ADF) protocol. Under this scheme, participants ate ad libitum on one day and 25% of maintenance caloric intake the next day. Briefly, what the study found is that a combination of cardiovascular exercise and ADF was more effective in reducing body weight and fat mass than either exercise or ADF alone. The combination group experienced a 6 ± 4 kg reduction in body weight vs. 3 ± 1 kg in the ADF-only group and 1 ± 1 kg in the exercise-only group. Improvements in various health biomarkers (↑HDL, ↓LDL, ↑LDL particle size) were observed in the combination group only.
The improvements seen in this study appeared in spite of questionable food choices. The menus on fasting days were:
Day 1 – Vegetarian pizza, apple, peanuts
Day 2 – Chicken enchilada, orange, crackers
Day 3 – Chicken fettuccini, carrot sticks, cookie
The estimated macronutrient ratios were 52% carbohydrate, 26% fat, and 22% protein. The training involved 40 minutes at 75% HRmax on either a stationary bike or elliptical machine.
Further studies must be done on a wider variety of populations and utilizing different intermittent fasting strategies, better quality food, and more effective exercise protocols. Available research suggests the fasting window is a powerful lever for driving change in both body composition and health. If you are currently eating for more than 12 hours a day, it would be worthwhile to experiment with a longer fasting window.
Additional Reading
Notes
- A widely known study indicated there is a maximum amount of protein that can be effectively absorbed after a workout. Over a 12-hour post-workout recovery period, the researchers compared eight doses of 10 g, four doses of 20 g, or two doses of 40 g of protein. Four doses of 20 g was found to have the most favorable increase in muscle protein synthesis. This led many to believe protein must be spread throughout the day for optimal effectiveness. However, this study used whey protein, which like all highly processed foods is rapidly absorbed. It is unlikely that the same is true for eggs, steak, or other unprocessed sources of protein, which take longer to digest, especially in the context of a balanced meal. The studies on time-restricted eating made waves for defying the conventional wisdom on both protein intake and meal frequency.