According to the carbohydrate-insulin model of obesity, high-carbohydrate diets increase hunger and low-carbohydrate diets reduce hunger due to their effects on insulin and glucagon. A high-carbohydrate diet will increase insulin and decrease glucagon. These changes suppress ketogenesis, lipolysis, and gluconeogenesis, thereby shifting available metabolic fuels toward storage rather than circulation or utilization. Conversely, a low-carbohydrate (or low-glycemic-index) diet will suppress insulin and increase glucagon levels with the opposite effects (1). This model is consistent with pharmacological data indicating drugs that raise the insulin-to-glucagon ratio are associated with weight gain, and those that reduce this ratio are associated with weight loss (2).
This 2020 study draws from the Framingham State Food Study (FS2), the primary results of which were previously summarized on CrossFit.com (3). Briefly, all subjects were fed a balanced, calorie-restricted diet to induce loss of 10-14% of their initial body weight. After a brief stabilization period, subjects were randomly assigned to a 20%-, 40%- or 60%-carbohydrate, weight-maintenance diet. During the extended weight-maintenance phase, subjects’ weights were continually monitored and their calorie intake adjusted to ensure weight stability — that is, if they gained weight, they were given fewer calories, and vice versa. All food was prepared in a metabolic kitchen and provided directly to subjects.
Between the 10th and 15th study weeks, subjects spent 24 hours in a metabolic ward at Brigham and Women’s Hospital, during which 24-hour metabolic measurements were taken. Subjects continued eating their study diets for this period.
For this study, subjects ate three meals during an overnight metabolic ward stay. Circulating glucose, ketone, fatty acid, and lactate levels were continually monitored. Researchers calculated the total circulating energy availability by summing the energy content of these three metabolic fuels.
As shown in Figure 1 below, mean levels of circulating energy availability did not differ significantly between subjects following a low-carb (LC), medium-carb (MC), or high-carb (HC) diet.
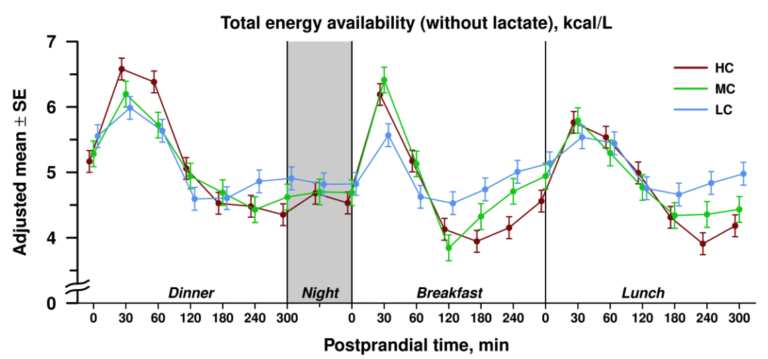
Figure 1: Total energy availability in subjects following high-, medium-, or low-carb diets
Instead, the three diets led to significantly different patterns of energy availability during the post-meal (postprandial) period. During the first 120 minutes after a meal, circulating energy availability was higher in subjects following an MC or HC diet than those following an LC diet. In the late postprandial period, however, the relationship reversed, with circulating energy availability higher in subjects following the LC diet than in subjects on the MC or HC diets. All differences noted were statistically significant.
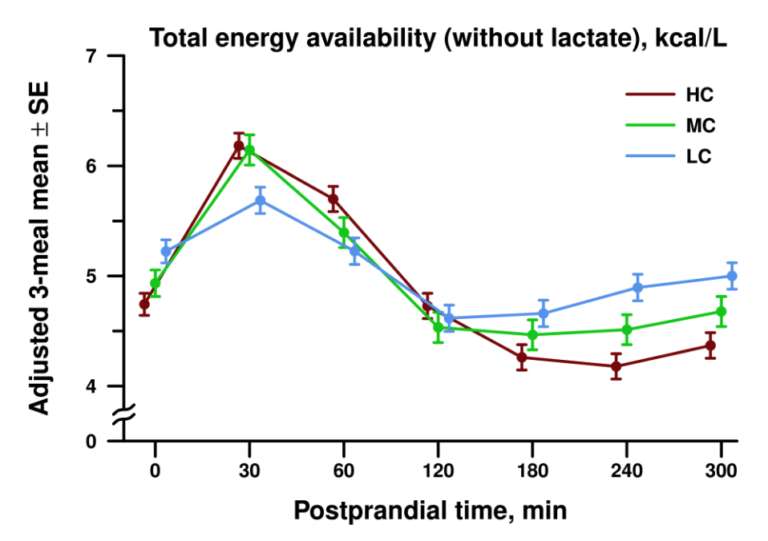
Figure 2: Mean postprandial energy availability in subjects following high-, medium-, or low-carb diets
The lower levels of circulating energy availability seen immediately after each meal in LC diet subjects (relative to MC and HC diet subjects) was primarily due to lower circulating glucose levels. The higher levels seen in the late postprandial period were primarily due to higher circulating fatty acid levels.
As expected, these differing patterns of metabolic fuel availability correlated with differing hormonal patterns. Total insulin AUC (area under the curve) was 64% higher in HC diet subjects than LC diet subjects; glucagon levels were higher at all times in LC diet than HC diet subjects. The mean postprandial insulin-to-glucagon ratio was higher by seven-fold in the HC group (2.5) than the LC group (0.36), reflecting an anabolic hormonal state.
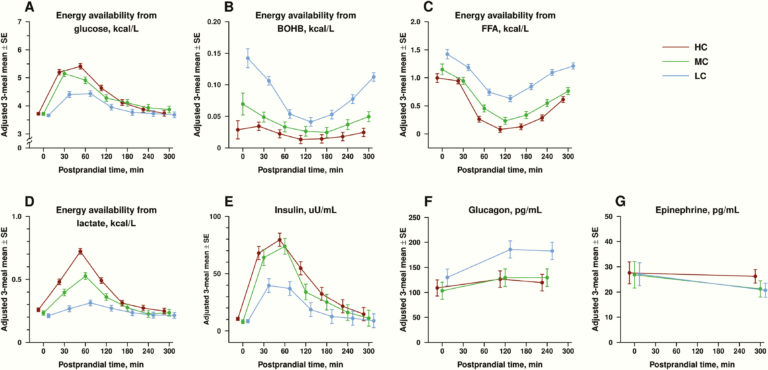
Figure 3: Circulating hormone and fuel levels in subjects following high-, medium-, or low-carb diets
These results are consistent with the predictions of the carb-insulin model of obesity. Increased dietary carbohydrate stimulates increased insulin release and decreased glucagon release, which drives a rapid reduction in circulating metabolic fuel levels. This model predicts lower circulating metabolic fuel levels will lead to greater post-meal hunger and, over time, greater caloric intake (4). Conversely, low-carbohydrate diets support extended elevation of circulating metabolic fuel levels and by this same logic would suppress hunger during weight loss or weight maintenance (5). In line with the broader results of the FS2 study, these results add to existing evidence suggesting carbohydrate restriction supports effective weight loss and maintenance.


