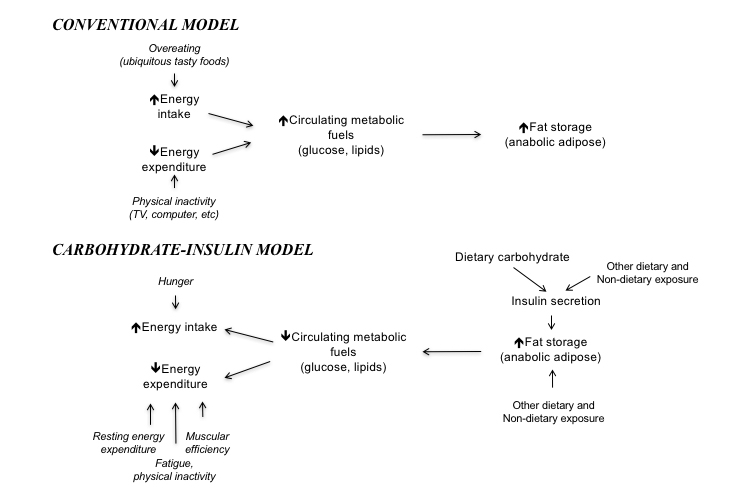
Takeaway: The carbohydrate-insulin model differs from the conventional model of obesity by showing a diet that increases insulin levels leads to fat accumulation independent of the number of calories it contains. Given the well-understood link between carbohydrate intake and insulin levels, this suggests carbohydrates, particularly highly refined carbohydrates and excess sugars, are calorie-for-calorie more obesogenic than other foods. Individuals who are relatively healthy may be able to lose weight merely by cutting these highly refined carbohydrates from their diet, as this change alone will lower insulin levels; those who are more unhealthy may have to cut more carbohydrates.
The Carbohydrate-Insulin Model of Obesity: Beyond "Calories In, Calories Out"
January 29, 2020
In this 2018 review, Drs. David Ludwig and Cara Ebbeling explain and provide support for the carbohydrate-insulin model of obesity (CIM).
In the carbohydrate-insulin model, the primary cause of obesity is found in the effect diet and other factors have on adipose (fat) tissue and the regulation of circulating fuels. Increasing insulin levels push circulating glucose out of the blood into storage by all tissues, including fat cells, while simultaneously suppressing the release of previously stored fatty acids by existing fat tissue. These and other effects lead insulin to shift consumed calories (energy) away from being used as fuel by lean tissues (e.g., organs, muscles, and the brain) and toward storage as glycogen and fat. In this model, the primary cause of obesity is an increase in insulin levels (either chronically or repeatedly), which leads to increased fat development, hunger, and reduced energy expenditure (1). Any diet, drug, or other factor that increases insulin levels can cause obesity.
Conversely, in the conventional model (CM) for obesity, all calories have similar effects with regard to obesity and fat development. Any diet in which the number of calories consumed (i.e., energy intake) exceeds the number of calories burned (i.e., energy expenditure) will lead to obesity; either increased food consumption or sedentary behavior is the cause of obesity. Within this model, a diet rich in refined sugars, processed carbohydrates, or other foods that stimulate insulin release is no more obesogenic than any other diet containing the same number of calories.
The two models are compared in the figure below.
Ludwig and Ebbeling observe that dosing insulin to treat Type 2 diabetes or providing excessive insulin for Type 1 diabetes is obesogenic while providing insufficient insulin or pharmacologically suppressing insulin leads to weight loss (2). Animal research has shown that injecting insulin into rodents increases fat deposition and hunger, and insulin-treated rats will put on fat even if fed a number of calories that leads to overall weight loss (3). Mice bred to produce less insulin are protected from diet-induced obesity even when overfed (4).
The CIM predicts a diet that increases insulin levels would also lead to obesity. Carbohydrate intake has a large impact on blood glucose and blood insulin levels while protein has a moderate impact and fat has very little impact. The CIM thus predicts a diet high in carbohydrate and low in fat would be expected to increase insulin levels and so lead to obesity. The glycemic index and glycemic load predict this postprandial (i.e., after-meal) insulin response accurately, with highly refined carbohydrates and sugars leading to higher glucose and insulin spikes (5).
Rodents fed high-glycemic diets become hyperinsulinemic and fatten even when calorically restricted (6). Short-term human feeding studies have shown high-glycemic-load meals increase hunger, decrease fat oxidation, and decrease energy expenditure in the hours immediately following the meal (7).
Two longer well-controlled human trials, Diogenes and DIRECT, found low-glycemic-load diets led to greater weight loss than high-glycemic-load diets (8). A third, DIETFITS, found no difference in weight loss induced by low-fat and low-carb diets (which differ dramatically in glycemic load), but both diets were designed to be “healthy” and therefore were absent any refined sugars or carbohydrates and low in glycemic load (9).
The metabolic benefits of carbohydrate restriction may increase over time, as the body takes two to three weeks to increase fat oxidation in response. Multiple studies have shown blood ketone levels continue to increase for at least three weeks after initiation of fasting or a ketogenic diet (10). After three weeks, nitrogen balance, initially negative on a low-carbohydrate diet, is normalized, indicating reduced reliance on protein as an energy source in the absence of carbohydrates (11).
The review authors note the impact of carbohydrate restriction will vary significantly between individuals. Some individuals naturally have a high insulin response to carbohydrate while others exhibit a lower response to the same foods. The CIM would expect the former group to especially benefit from carbohydrate restriction while the latter group would see relatively smaller differences between the effects of high-carbohydrate and low-carbohydrate diets (12).
In sum, the carbohydrate-insulin model suggests foods that elevate insulin shuttle metabolic fuels away from usage and toward storage, simultaneously increasing hunger, decreasing energy expenditure, and increasing fat storage. The cited literature shows this model is consistent with the observed differences between diets that differ in glycemic index and glycemic load and indicates reducing diet-induced insulin elevation will support weight loss.
Notes
- The glycemic index: Physiological mechanisms relating to obesity, diabetes and cardiovascular disease; Increasing adiposity: Consequence or cause of overeating?; The science of obesity: What do we really know about what makes us fat?
- Inhibition of insulin secretion as a new drug target in the treatment of metabolic disorders; Intensive insulin therapy and weight gain in IDDM
- Hyperinsulinemia and its impact on obesity and insulin resistance; Elevated insulin and satiety in obese and normal-weight rats; Insulin increases body fat despite control of food intake and physcial activity
- Hyperinsulinemia drives diet-induced obesity independently of brain insulin production
- Prediction of glucose and insulin responses of normal subjects after consuming mixed meals varying in energy, protein, fat, carbohydrate and glycemic index
- Dietary amylose-amylopectin starch content affects glucose and lipid metabolism in adipocytes of normal and diabetic rats; Effects of long-term low-glycemic index starchy food on plasma glucose and lipid concentrations and adipose tissue cellularity in normal and diabetic rats; A high glycemic index starch diet affects lipid storage-related enzymes in normal and to a lesser extent in diabetic rats; Effects of dietary glycemic index on adiposity, glucose homeostasis, and plasma lipids in animals
- A low-glycemic diet lifestyle intervention improves fat utilization during exercise in older obese humans; Effects of diet compsition on postprandial energy availability during weight loss maintenance
- Diets with high or low protein content and glycemic index for weight-loss maintenance; Weight loss with a low-carbohydrate, mediterranean or low-fat diet
- Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: The DIETFITS randomized clinical trial
- Ketosis of starvation: A revisit and new perspectives; Composition of weight lost during short-term weight reduction. Metabolic responses of obese subejcts to starvation and low-calorie ketogenic and nonketogenic diets
- Protein sparing during treatment of obesity: Ketogenic versus non ketogenic very low calorie diet
- A novel interaction between dietary composition and insulin secretion: Effects on weight gain in the Quebec Family Study; Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial; A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial; Impact of dietary glycemic challenge on fuel partitioning