The Current Cancer Crisis
Over 1,600 people die each day from cancer in the U.S., according to recent data from the American Cancer Society (1). The 4.8 percent increase in death rate from cancer over the last five years is greater than the 4.2 percent increase in U.S. population growth over the same period.
In this context, it is important to take a close look at both the prevailing and alternative theories of cancer’s origin. Over the next several weeks, we will review the history of and evidence for the somatic mutation theory (SMT) and the mitochondrial metabolic theory (MMT) of cancer.
The Somatic Mutation Theory Persists as the Dominant Explanation for the Origin of Cancer
The prevailing view today is that cancer is a “genetic disease” involving nuclear mutations in oncogenes and tumor suppressor genes (2-4). A typical tumor is thought to contain several so-called “driver gene” mutations that regulate the tumorigenic phenotype (5,6). The nuclear genomic instability seen in nearly all types of tumor cells is considered the primary cause of the cancer’s hallmarks, which include sustained proliferative signaling, evasion of growth suppressors, resistance to cell death, replicative immortality, enhanced vascularization, and activation of invasion and metastasis (2). Somatic mutations, which arise randomly during DNA replication in normal non-cancerous stem cells, are considered the origin of cancer (7).
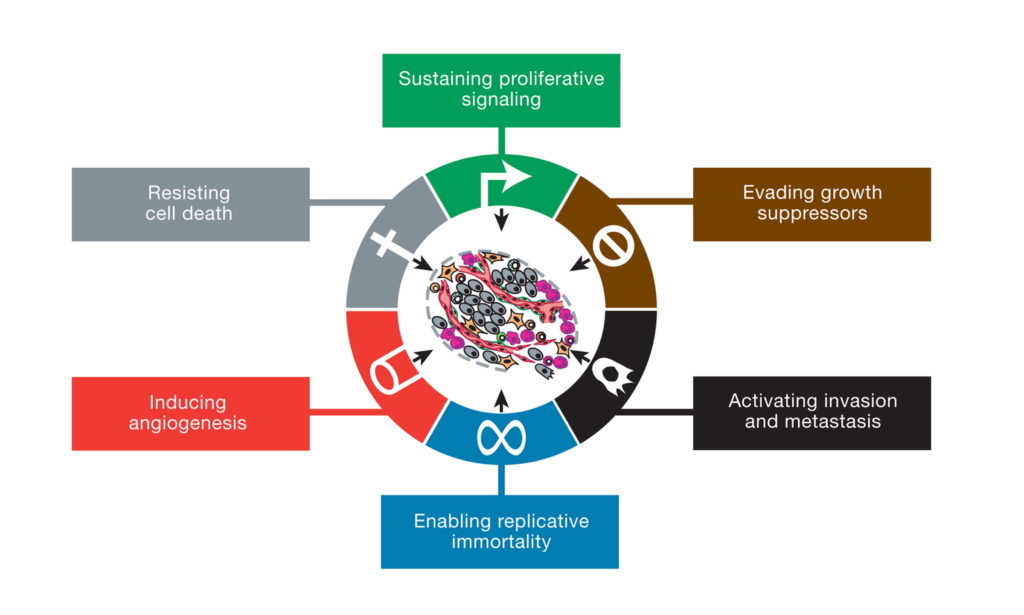
This illustration encompasses the six hallmark capabilities of cancer, originally proposed in 2000 and since substantiated with metabolic underpinnings. From (2).
The somatic mutation theory reigns as the most widely accepted view of the origin of cancer and is the justification for developing personalized genetic therapies or precision medicine for managing the various forms of the disease (4, 8-10). The theory is presented as proven law in most current college textbooks of genetics, biochemistry, and cell biology and is the mainstay of the multi-billion-dollar cancer industry and of the National Cancer Institute (NCI). The NCI website states, “Cancer is a genetic disease—that is, it is caused by changes to genes that control the way our cells function, especially how they grow and divide.” The cancer drug industry and the NIH both consider cancer to be a genetic disease.
The Mitochondrial Metabolic Theory of Cancer
Although the SMT is currently the dominant theory for the origin of cancer, the mitochondrial metabolic theory is emerging as an alternative explanation. Mitochondria are the organelles that produce most cellular energy and are largely responsible for maintaining metabolic homeostasis of the body. The MMT originated with the work of Otto Warburg in the last century and has been resurrected more recently by the work of Seyfried and others (11-17).
The MMT argues that cancer arises primarily from defects in energy production through oxidative phosphorylation (OxPhos) in the mitochondria. OxPhos generates the majority of energy for most cells of the body. Defects in the number, structure, and function of mitochondria will cause cells to gradually replace insufficient respiration with fermentation for energy production, thus initiating the path to neoplasia. Aerobic fermentation of lactic acid, also called the Warburg effect, is recognized as the most common pathological phenotype of cancer. Recent evidence also shows that tumor cells can also use mitochondrial substrate level phosphorylation as another fermentation pathway to compensate for defective respiration. Mitochondrial substrate level phosphorylation is now recognized as the “missing link” in Warburg’s central theory (18). Defective OxPhos with a compensatory reliance on fermentation for energy produces reactive oxygen species (ROS) that are both mutagenic and carcinogenic. According to the MMT, the somatic mutations and all other hallmarks of cancer are considered downstream epiphenomenon for the initial damage to respiration. Hence, the MMT differs from the EMT in placing the origin of the disease in the mitochondria rather than in the nucleus of the cell.
