Science Challenging the Somatic Mutation Theory: Concerns Regarding the Epithelial Mesenchymal Transition (EMT) as an Explanation for Metastasis
Metastasis involves the spread of tumor cells from a primary site to distant sites with continued relentless growth. Metastasis is responsible for about 90 percent of cancer deaths and is the most fearsome aspect of cancer. Stephen Paget first proposed that metastasis depends on cross-talk between selected cancer cells (the seeds) and specific microenvironments (the soil). His concept still holds true today, according to Dr. Isaiah Fidler, a leader in cancer metastasis (36).
The epithelial mesenchymal transition (EMT) is currently the dominant explanation for the origin of cancer metastasis and is based on the somatic mutation theory of cancer (SMT). According to the EMT, random somatic mutations cause normal columnar epithelial cells to transform into non-adherent mesenchymal cells. These mutated mesenchymal cells then invade through the local tissue, intravasate (enter) blood vessels, survive in the circulation, suppress the immune system, extravasate (leave) blood vessels, and establish growth at sites distant from the primary tumor. No explanation is provided for how tumor cells with deranged genomes and random mutations could metastasize in a non-random manner according to the seed-soil hypothesis. David Tarin also believes that the EMT is fiction, as it has never been observed in cancer pathological specimens (37).
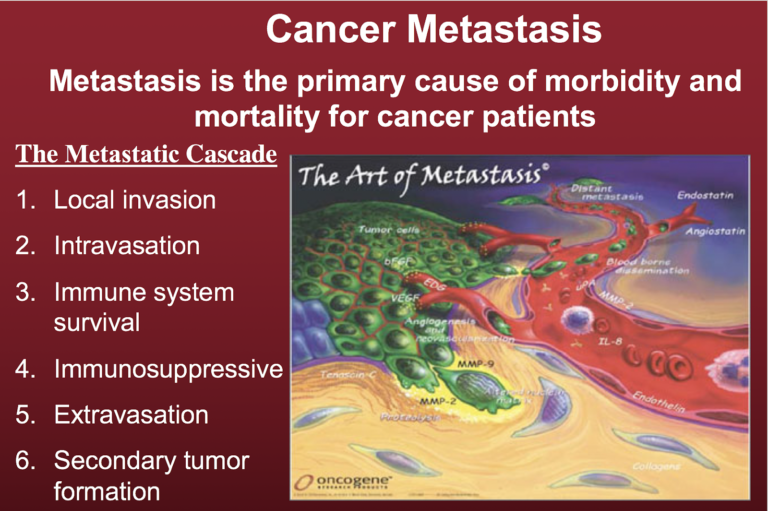
Figure 1 illustrates the well-documented metastatic cascade. A key aspect of metastasis is the non-random manner by which the metastatic cancer cells invade distant organs and tissues, with lung, liver, and bone as some of the more preferred sites. The green cells shown in Figure 1 are the tumor cells.
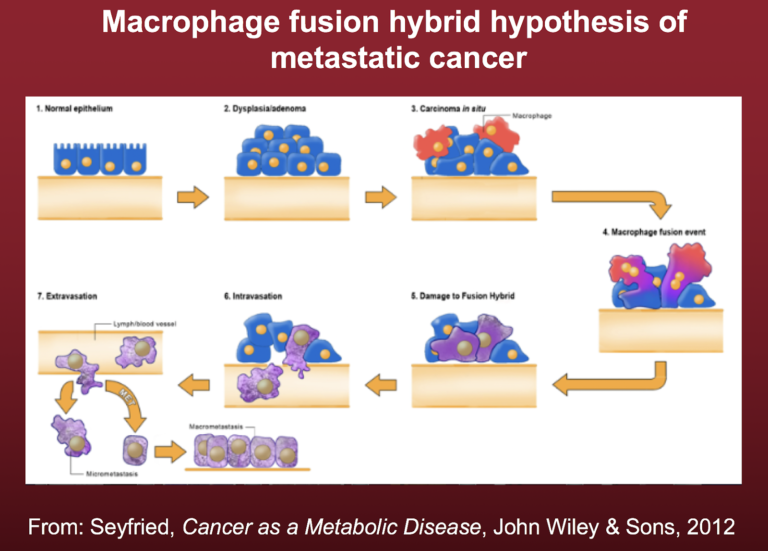
Emerging evidence indicates that metastatic cancer cells arise following the fusion of cancer stem cells with macrophages. Macrophages are already mesenchymal cells and are capable of entering and exiting tissues. Recent studies from John Pawelek, Melissa Wong, and Ivan Shabo provide compelling evidence that metastatic cancer cells arise from fusion hybridization of cancer cells and macrophages (38-40). We suggested that the replacement of normal mitochondria with defective mitochondria in the fusion hybrids could link the fusion hybrid hypothesis to the mitochondrial metabolic theory of cancer (MMT) (41). The process is illustrated in Figure 2.
Figure 2 depicts the origin of metastatic cancer cells, wherein the fusion event creates rogue macrophage-type cells with the capacity to invade through local tissue, intravasate (enter) blood vessels, survive in the circulation, suppress the immune system, extravasate (leave) blood vessels, and finally establish growth at sites distant from the primary tumor. The fusion hybrid hypothesis linked with the MMT can better explain the origin of cancer metastasis than can the EMT.
It is important to note that the fusion hybrid hypothesis originated with the work of the German pathologist Otto Aichel in 1911 (42). Aichel actually saw macrophage-type cells fusing with cancer cells under the microscope, providing direct evidence for the fusion event. Interestingly, Theodor Boveri crafted his chromosome mutation theory based on Aichel’s findings, as Aichel also noted abnormal chromosomes in the fused hybrids. It is therefore remarkable that the results from a single observation of cancer tissue would lead eventually to two fundamentally different explanations for the origin of cancer metastasis.
Related
- Is Cancer a Genetic or Metabolic Disease? Part 1
- Is Cancer a Genetic or Metabolic Disease? Part 2
- Is Cancer a Genetic or Metabolic Disease? Part 3
Thomas N. Seyfried is professor of biology at Boston College. He received a doctorate in genetics and biochemistry from the University of Illinois—Urbana-Champaign in 1976. He did his undergraduate work at the University of New England, where he recently received the distinguished Alumni Achievement Award. He also holds a master’s degree in genetics from Illinois State University. Seyfried served with distinction in the United States Army’s 1st Cavalry Division during the Vietnam War and received numerous medals and commendations.
He was a postdoctoral fellow in the Department of Neurology at the Yale University School of Medicine and then served on the faculty as an assistant professor in neurology. Seyfried previously served as chair of the Scientific Advisory Committee for the National Tay-Sachs and Allied Diseases Association. He recently received a Lifetime Achievement Award from the Academy of Complementary and Integrative Medicine and the Uncompromising Science Award from the American College of Nutrition for his work on cancer.
He presently serves on several editorial boards, including those for Nutrition & Metabolism, Neurochemical Research, the Journal of Lipid Research, and ASN Neuro. Seyfried has over 180 peer-reviewed publications and is author of the book “Cancer as a Metabolic Disease: On the Origin, Management, and Prevention of Cancer” (Wiley Press).
References
Note: These references include those previously published in “Is Cancer a Genetic or Metabolic Disease? Part 1,” “Part 2” and “Part 3.”
- Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 68.1(2018): 7-30. Available here.
- Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. Cell 144.5(2011): 646-674. Available here.
- Vogelstein B, Papadopoulos N, Velculescu VE et al. Cancer genome landscapes. Science 339.6127(2013): 1546-1558. Available here.
- Hou JP and Ma J. DawnRank: discovering personalized driver genes in cancer. Genome Medicine 6.7(2014): 56. Available here.
- Iranzo J, Martincorena I, and Koonin EV. Cancer-mutation network and the number and specificity of driver mutations. Proceedings of the National Academy of Sciences of the United States of America 115.26(2018): E6010-E6019. Available here.
- Fearon ER and Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 61.5(1990): 759-767. Available here.
- Tomasetti C and Vogelstein B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347.6217(2015): 78-81. Available here.
- Vaux DL. In defense of the somatic mutation theory of cancer. BioEssays 33.5(2011): 341-343. Available here.
- McLeod HL. Cancer pharmacogenomics: early promise, but concerted effort needed. Science 339.6127(2013): 1563-1566. Available here.
- Ju J, Zhu A, and Yuan P. Progress in targeted therapy for breast cancer. Chronic Diseases and Translational Medicine 4.3(2018): 164-175. Available here.
- Seyfried TN. Cancer as a Metabolic Disease: On the Origin, Management and Prevention of Cancer. Hoboken, New Jersey: John Wiley & Sons, Inc., 2012. Available here.
- John AP. Dysfunctional mitochondria, not oxygen insufficiency, cause cancer cells to produce inordinate amounts of lactic acid: the impact of this on the treatment of cancer. Medical Hypotheses 57.4(2001): 429-431. Available here.
- Kim A. Mitochondria in cancer energy metabolism: culprits or bystanders? Toxicological Research 31.4(2015): 323-330. Available here.
- Pelicano H, Zhang W, Liu J et al. Mitochondrial dysfunction in some triple-negative breast cancer cell lines: role of mTOR pathway and therapeutic potential. Breast Cancer Research 16.5(2014): 434. Available here.
- Srinivasan S, Guha M, Dong DW et al. Disruption of cytochrome c oxidase function induces the Warburg effect and metabolic reprogramming. Oncogene 35.12(2016): 1585-1595. Available here.
- Stefano GB and Kream RM. Cancer: mitochondrial origins. Medical Science Monitor 21(2015): 3736-3739. Available here.
- Warburg O. On the origin of cancer cells. Science 123.3191(1956): 309-314. Available here.
- Chinopoulos C and Seyfried TN. Mitochondrial substrate-level phosphorylation as energy source for glioblastoma: review and hypothesis. ASN Neuro 10(2018): 1-27. Available here.
- Boveri T. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. Journal of Cell Science 121. Suppl.1(2008): 1-84. Available here.
- Nowell PC. The clonal evolution of tumor cell populations. Science 194.4260(1976): 23-28. Available here.
- Weinberg RA. The Biology of Cancer. New York City, New York: Garland Science, 2007. Available here.
- Darlington CD. The plasmagene theory of the origin of cancer. British Journal of Cancer 2.2(1948): 118-126. Available here.
- Rous, P. Surmise and fact on the nature of cancer. Nature 183(1959): 1357-1361. Available here.
- Baker SG and Kramer BS. Paradoxes in carcinogenesis: new opportunities for research directions. BMC Cancer 7(2007): 151. Available here.
- Soto AM and Sonnenschein C. The somatic mutation theory of cancer: growing problems with the paradigm? Bioessays 26.10(2004): 1097-1107. Available here.
- Sonnenschein C and Soto AM. Somatic mutation theory of carcinogenesis: why it should be dropped and replaced. Molecular Carcinogenesis 29.4(2000): 205-211. Available here.
- Burgio E and Migliore L. Towards a systemic paradigm in carcinogenesis: linking epigenetics and genetics. Molecular Biology Reports 42.4(2015): 777-790. Available here.
- Seyfried TN. Cancer as a mitochondrial metabolic disease. Frontiers in Cell and Developmental Biology 3(2015): 43. Available here.
- Li L, Connelly MC, Wetmore C et al. Mouse embryos cloned from brain tumors. Cancer Research 63.11(2003): 2733-2736. Available here.
- McKinnell RG, Deggins BA and Labat DD. Transplantation of pluripotential nuclei from triploid frog tumors. Science 165.3891(1969): 394-396. Available here.
- Hochedlinger K, Blelloch R, Brennan C et al. Reprogramming of a melanoma genome by nuclear transplantation. Genes & Development 18.15(2004): 1875-1885. Available here.
- McNutt M. Journals unite for reproducibility. Science 346.6210(2014): 679. Available here.
- Baker M. 1,500 scientists lift the lid on reproducibility. Nature 533.7604(2016): 452-454. Available here.
- Nosek BA and Errington TM. Making sense of replications. eLife 6: e23383, 2017. Available here.
- Sonnenschein C and Soto AM. Theories of carcinogenesis: an emerging perspective. Seminars in Cancer Biology 18.5(2008): 372-377. Available here.
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature Reviews Cancer 3.6(2003): 453-458. Available here.
- Tarin D. Cell and tissue interactions in carcinogenesis and metastasis and their clinical significance. Seminars in Cancer Biology 21.2(2011): 72-82. Available here.
- Garvin S, Oda H, Arnesson LG et al. Tumor cell expression of CD163 is associated to postoperative radiotherapy and poor prognosis in patients with breast cancer treated with breast-conserving surgery. Journal of Cancer Research and Clinical Oncology 144.7(2018): 1253-1263. Available here.
- Gast CE, Silk AD, Zarour L et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Science Advances 4.9(2018): eaat7828. Available here.
- Pawelek JM and Chakraborty AK. The cancer cell–leukocyte fusion theory of metastasis. Advances in Cancer Research 101(2008): 397-444. Available here.
- Seyfried TN and Huysentruyt LC. On the origin of cancer metastasis. Critical Reviews in Oncogenesis 18.1-2(2013): 43-73. Available here.
- Pawelek JM and Chakraborty AK. Fusion of tumour cells with bone marrow-derived cells: a unifying explanation for metastasis. Nature Reviews Cancer 8.5(2008): 377-386. Available here.
- Chernet BT, Fields C and Levin M. Long-range gap junctional signaling controls oncogene-mediated tumorigenesis in Xenopus laevis embryos. Frontiers in Physiology 5(2015): 519. Available here.
- Chernet BT and Levin M. Transmembrane voltage potential of somatic cells controls oncogene-mediated tumorigenesis at long-range. Oncotarget 5.10(2014): 3287-3306. Available here.
- Baker SG. A cancer theory kerfuffle can lead to new lines of research. JNCI Journal of the National Cancer Institute 107.2(2015): dju405. Available here.
- Kiebish MA and Seyfried TN. Absence of pathogenic mitochondrial DNA mutations in mouse brain tumors. BMC Cancer 5(2005): 102. Available here.
- Parsons DW, Jones S, Zhang X et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321.5897(2008): 1807-1812. Available here.
- Martincorena I and Campbell PJ. Somatic mutation in cancer and normal cells. Science 349.6255(2015): 1483-1489. Available here.
- Martincorena I, Fowler JC, Wabik A et al. Somatic mutant clones colonize the human esophagus with age. Science 362.6417(2018): 911-917. Available here.
- Nishioka M, Bundo M, Iwamoto K et al. Somatic mutations in the human brain: implications for psychiatric research. Molecular Psychiatry 2018. Available here.
- Gauci ML, Boudou P, Baroudjian B et al. Occurrence of type 1 and type 2 diabetes in patients treated with immunotherapy (anti- PD-1 and/or anti-CTLA-4) for metastatic melanoma: a retrospective study. Cancer Immunology, Immunotherapy 67.8(2018): 1197-1208. Available here.
- Stupp R, Hegi ME, Mason WP et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology 10.5(2009): 459-466. Available here.
- Johnson BE, Mazor T, Hong C et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 343.6167(2014): 189-193. Available here.
- Seyfried TN, Flores R, Poff AM et al. Metabolic therapy: a new paradigm for managing malignant brain cancer. Cancer Letters 356. 2 Pt. A(2015): 289-300. Available here.
- Seyfried TN, Yu G, Maroon JC and D’Agostino DP. Press-pulse: a novel therapeutic strategy for the metabolic management of cancer. Nutrition and Metabolism 14.19(2017). Available here.
All links accessed Feb. 7, 2019.