This 2017 review summarizes multiple mechanisms by which insulin resistance and metabolic dysfunction could drive development and/or progression of Alzheimer’s disease.
Current evidence suggests hyperinsulinemia, and/or insulin resistance specifically, is most strongly linked to increased risk of Alzheimer’s. The link between obesity and Alzheimer’s disease is more tenuous, with some studies showing no increased risk of cognitive impairment due to obesity. Conversely, Alzheimer’s patients show higher levels of fasting insulin than controls, and individuals with diabetes and/or metabolic syndrome show consistently elevated Alzheimer’s risk (2).
Insulin is known to play a role in brain function, with insulin receptors expressed throughout the brain and insulin activity greatest in areas associated with cognition, sensation, and memory formation (3). Insulin regulates the expression of neurotransmitter receptors and therefore neuroplasticity and synapse function (4).
Insulin levels in the brain, however, do not correlate with insulin levels in the rest of the body. Insulin must be actively transported across the blood-brain barrier (5), and this barrier can become insulin resistant. Chronic hyperinsulinemia can thus lead to simultaneously high levels of insulin in the periphery and low levels in the brain and cerebrospinal fluid, a pattern frequently seen in patients with Alzheimer’s (6).
Alzheimer’s frequently has been linked to accumulation of amyloid beta (AB) proteins, with patients exhibiting elevated levels of AB in the brain and cerebrospinal fluid (7). Insulin-degrading enzyme (IDE) plays a major role in breaking down AB oligomers (i.e., the precursor to harmful amyloid plaques), and the expression of IDE is correlated with the level of insulin activity in the brain (7). As a result, decreased insulin activity, via its effect on IDE, contributes to AB accumulation. As AB accumulates, it competes with insulin for access to insulin receptors; the result is a vicious cycle in which decreased insulin activity leads to AB accumulation, which leads to a further decrease in insulin activity (8).
Multiple rodent studies have found that inducing insulin resistance or suppressing insulin activity in the brains of rats and mice leads to cognitive impairment resembling Alzheimer’s (9). These observations, which consistently point to the role of insulin resistance in the genesis and/or progression of Alzheimer’s, have led some to call Alzheimer’s “Type 3 diabetes” (10).
Cerebral hypometabolism — that is, decreased energy production in the brain specifically due to reduced glucose metabolism — is also linked to metabolic dysfunction and Alzheimer’s disease. Glucose uptake in the brain is insulin stimulated, so impairments in insulin signaling lead to decreased glucose uptake and metabolism alongside their direct effects on cognition (11). Defective glucose metabolism, especially within the hippocampus and other areas associated with memory formation and cognition, is one of the first signs of Alzheimer’s, appearing in high-risk patients 20-30 years prior to other symptoms (12). As AD progresses, the degree of cerebral glucose hypometabolism correlates with the degree of cognitive impairment observed (13). Inflammation and oxidation, themselves consequences of metabolic syndrome systemically and decreased energy and insulin availability locally, have also been linked to amyloid accumulation and other symptoms of Alzheimer’s (14). In particular, some research has suggested defects in mitochondrial structure, which simultaneously increase the production of reactive oxygen species and decrease their clearance, may play a role in Alzheimer’s progression (15).
Overall, the hypothesis that Alzheimer’s is a metabolic disease remains preliminary and supported by limited evidence in humans beyond epidemiological correlations. The evidence and mechanisms that have been proposed, however, clearly indicate how metabolic defects — including insulin resistance, inflammation, oxidation, and impairments to metabolism in the brain — could explain both the genesis and development of Alzheimer’s disease. The multiple mechanisms by which insulin resistance can affect Alzheimer’s progression are summarized in the figure below.
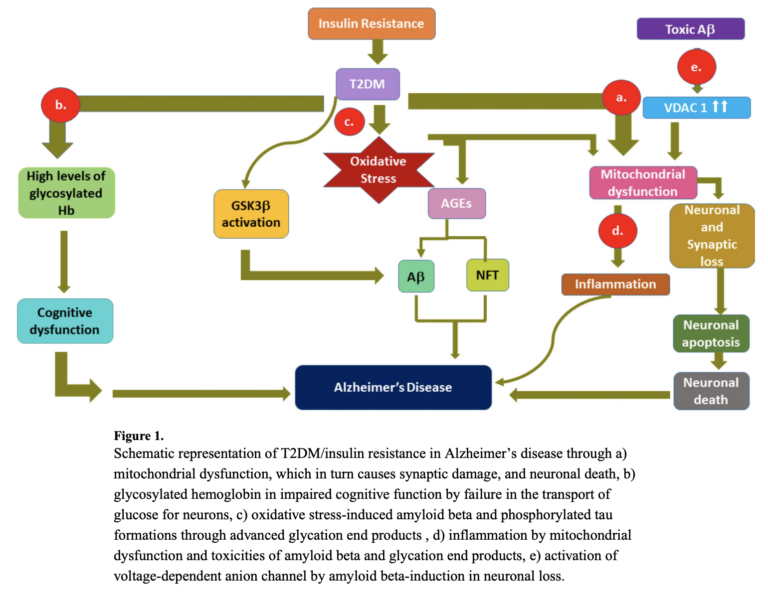
Takeaway: Accumulating evidence suggests diabetes and other forms of metabolic dysfunction exacerbate and may even cause Alzheimer’s disease and cognitive impairment.
Notes
- The insulin signalling pathway; GSK3 inhibitors: Development and therapeutic potential; Early onset of cognitive impairment is associated with altered synaptic plasticity and enhanced hippocampal GluA1 expression in a mouse model of depression; The epidemiology of adiposity and dementia; Midlife and late-life obesity and the risk of dementia: Cardiovascular health study; Measures of adiposity and dementia risk in elderly persons
- Molecular links between Alzheimer’s disease and diabetes mellitus; Cognitive decline in dementia and diabetes – systematic overview of prospective observational studies; The effects of type 1 diabetes on cognitive performance: A meta-analysis; Type 2 diabetes mellitus. Cognitive impairment and dementia; Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies; Hyperinsulinemia and risk of Alzheimer disease; Diabetes impaired fasting glucose and development of cognitive impairment in older women; Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheiemer’s disease; Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: Cross sectional population based study; Hyperinsulinemia and risk of Alzheimer disease; Insulin metabolism and the risk of Alzheimer disease: The Rotterdam study; Fasting insulin and incident dementia in an elderly population of Japanese-American men; Hyperinsulinemia and Alzheimer’s disease; Obesity insulin resistance and Alzheimer’s disease
- Molecular connexions between dementia and diabetes; Cognitive effects of insulin in the central nervous system; Tracking insulin to the mind; Role of insulin and insulin receptor in learning and memory
- Early onset of cognitive impairment is associated with altered synaptic plasticity and enhanced hippocampal GluA1 expression in a mouse model of depression; Insulin can induce the expression of a memory-related synaptic protein through facilitating AMPA receptor endocytosis in rat cortical neurons; An investigation into signal transduction mechanisms involved in insulin-induced long-term depression in the CA1 region of the hippocampus; The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses; Insulin causes a transient tyrosine phosphorylation of NR2A and NR2B NMDA receptor subunits in rat hippocampus, etc.
- Insulin receptors and insulin action in the brain: review and clinical implications
- Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: Relationship to severity of dementia and apolipoprotein E genotype
- Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation; Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme
- Amyloid beta oligomers induce impairment of neuronal insulin receptors; Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by A beta oligomers
- Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats; Contributions of brain insulin resistance and deficiency in amyloid-related neurodegeneration in Alzheimer’s disease; Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein; Inhibition of the neuronal insulin receptor. An in vivo model for sporadic Alzheimer disease?; Metabolic changes in rat brain following intracerebroventricular injections of streptozotocin: a model of sporadic Alzheimer’s disease.; Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease
- Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine; Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s disease; Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes?; Type 3 diabetes is sporadic Alzheimer’s disease: Mini-review
- Review of insulin insulin-like growth factor expression, signaling and malfunction in the central nervous system: Relevance to Alzheimer’s disease; Intranasal insulin improves memory in humans; Effects of intranasal insulin on cognition in memory-impaired older adults: Modulation by APOE genotype; Intranasal insulin improves cognition and modulates beta-amyloid in early AD; High carbohydrate diets and Alzheimer’s disease; Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein; Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia; Altered cerebral energy metabolism in Alzheimer’s disease: A PET study; Insulin signaling glucose metabolism and mitochondria: major players in Alzheimer’s disease and diabetes interrelation; Hippocampal hypometabolism predicts cognitive decline from normal aging
- Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia
- Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment; Hypometabolism in Alzheimer-affected brain regions in cognitively healthy Latino individuals carrying the apolipoprotein E epsilon4 allele; Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease; FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease; Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: therapeutic implications; Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: a cross-sectional comparison against advanced late-onset and incipient early-onset cases
- Inflammatory pathways and insulin action; Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment?; Early inflammation and dementia: A 25-year follow-up of the Honolulu-Asia aging study; Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study
- Alzheimer’s disease is type 3 diabetes-evidence reviewed; Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease; Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: Implications for free radical generation and oxidative damage in disease progression; Molecular linkages between diabetes and Alzheimer’s disease: current scenario and future prospects; The role of insulin resistance in the pathogenesis of Alzheimer’s disease: Implications for treatment
Comments on Is Alzheimer’s Disease a Type 3 Diabetes? A Critical Appraisal
I remember back in the day when it was thought that aluminum in cookware and antiperspirants was the causative agent of Alzheimer’s disease. It doesn’t seem like treatment outcomes have improved greatly since then, so it’s good to see new ideas are being considered.
That scientists are considering the “type 3 diabetes” moniker signals a pretty major shift in thinking on the disease. Their proposed mechanism is compelling- that insulin resistance of the blood brain barrier could lead to hypometabolism and then cell damage. One interesting thing to me is that relatively large neurons, which are the most metabolically active are also most impaired by Alzheimer’s.
Getting off the carbs and the couch seems likely to hold more promise than doing crossword puzzles and foregoing deodorant.
Is Alzheimer’s Disease a Type 3 Diabetes? A Critical Appraisal
1