This brief 2013 review published in Nature summarizes two studies investigating the metabolic impact of fructose overconsumption, placing them in the broader context of the scientific literature.
Fructose is mechanistically linked to liver fat accumulation. Unlike glucose, fructose can be converted into fat in the liver in a largely unrestricted fashion, and thus high fructose diets can lead to overproduction of liver fat. At the same time, this fat can be released into circulation and end up in other tissues, causing insulin resistance and metabolic diseases such as cardiovascular disease, fatty liver disease, and Type 2 diabetes. These processes, as the authors note, have also been linked to a wide range of cancers.
The authors of the review summarize two mouse studies, both of which used mice that were unable to metabolize fructose and thus immune to its effects on liver fat. These mutated mice were resistant to fatty liver disease, even when fed diets shown to lead to fatty liver disease in wild-type (i.e., normal) mice. The results suggest fructose plays a unique role in liver fat accumulation, beyond other carbohydrates or calories.
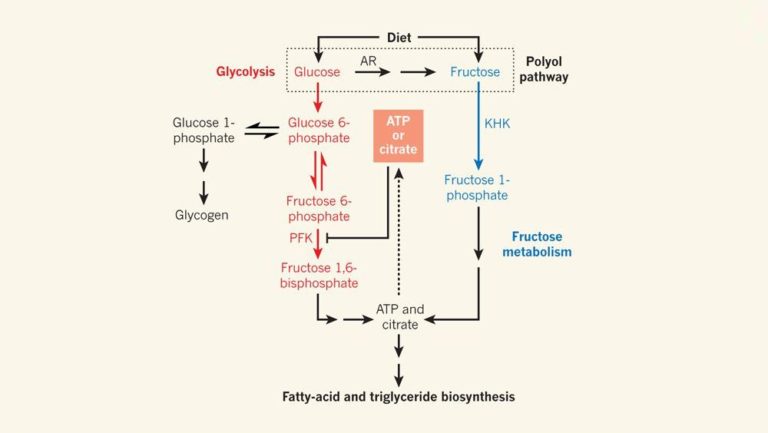
Figure 1: Sugar Metabolism.
Glucose and fructose are dietary sugars. Glucose is mainly metabolized by glycolysis and can be regulated through feedback inhibition by ATP or citrate, which redirects glucose towards storage as glycogen. In liver cells expressing the enzyme ketohexokinase (KHK), fructose is metabolized to fatty acids, triglyceride head groups (glycerol, not shown) and triglycerides — a biosynthetic pathway that is not regulated by the ATP- or citrate-feedback mechanisms. Glucose can also be converted into fructose through the polyol pathway, which involves the action of the enzyme aldose reductase (AR). In the absence of feedback inhibition, glucose metabolites can also be used to generate fatty acids and triglycerides. PFK, phosphofructokinase.
The authors note this fat accumulation mechanism may have been advantageous at one point in humanity’s past—as fruits ripen at the end of a growing season, starches convert to fructose, and fructose metabolism could have facilitated fat storage through the subsequent, calorie-deprived months. In our current context, however, where fructose-rich foods are readily available all year, this process has become maladaptive.
The authors conclude:
Whether fructose biosynthesis from a high-glucose diet is also relevant to metabolic syndrome in humans remains unknown. Regardless of the answer, the current papers make a strong case for the toxic effects of excess carbohydrate intake, placing fructose metabolism in the crosshairs.